Describe Using a Chemical Equation the Breakdown of Hydrogen Peroxide
Reaction of the breakdown of hydrogen peroxide and its features Properties of hydroperite aka perhydrol. Write the balanced equation for the breakdown of hydrogen peroxide.
How To Balance H2o2 O2 H2o Decomposition Of Hydrogen Peroxide Youtube
How does hydrogen peroxide decompose.

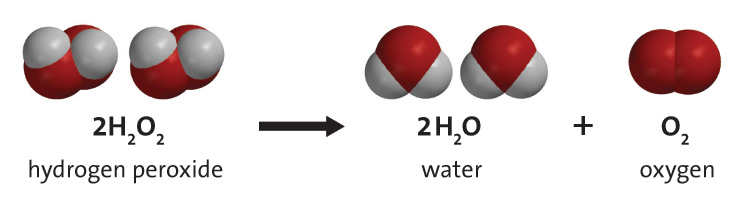
. The following reactions depict the picture. 2H 2 O 2aq- 2H 2 O l O 2g. Demonstrate the effectiveness of different catalysts for the decomposition of hydrogen peroxide by assessing the reaction rate of varying mixtures of hydrogen peroxide washing up liquid and a selection of catalysts.
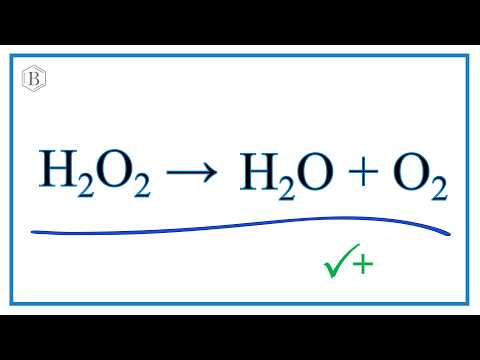
2H 2 O 2 aq O 2 g 2H 2 Ol. However this was not the exact reaction that took place. However to make the reaction perceptible at a.
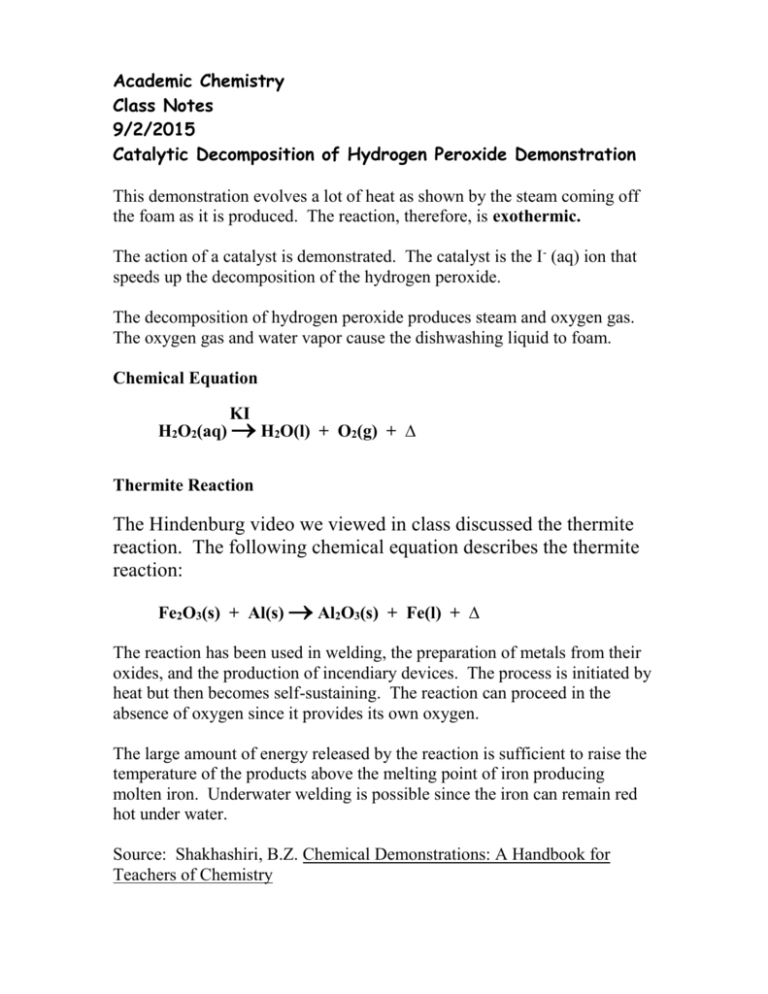
H 2 O 2 2H 2e- O 2 Acidic medium H 2 O 2 2OH 3H 2 O 2e- O 2 Basic medium 7. Otherwise known as a peroxide bond this is incredibly weak and unstable. Hydrogen peroxide reaction equation Decomposition H2O2-- H2O O2.
The resulting products are water and oxygen gas. On a large sheet of plastic backed absorbent paper place a 1 liter graduated cylinder on a tray or large petrie dish. Oxidizing nature in an acidic medium.
BaO 2 8H 2 Os H 2 SO 4 aq BaSO 4 s H 2 O 2 aq 8H 2 Ol. Hydrogen peroxide is a chemical compound with the formula H 2 O 2In its pure form it is a very pale blue liquid slightly more viscous than waterIt is used as an oxidizer bleaching agent and antiseptic usually as a dilute solution 36 by weight in water for consumer use and in higher concentrations for industrial useConcentrated hydrogen peroxide or high-test peroxide. Add 50 mL 30 Hydrogen Peroxide and 4 drops of dish soap to the graduated cylinder.
Thats why its stored in dark containers. Hydrogen peroxide oxygen water. The following reaction will clarify this.
The balanced equation of the decomposition reaction of hydrogen peroxide is that 2H2O2 decomposes into the products 2H2O O2 g. Show the fully labeled balanced chemical equation for the decomposition of hydrogen peroxide. You will see bubbling when the meat has the hydrogen peroxide placed on it.
In pure form it is unstable and easily breaks down with the process often being accompanied by. Light and temperature affect the reaction rate. When manganese dioxide is added to a hydrogen peroxide solution in water is catalyzes the breakdown of the hydrogen peroxide into water and oxygen gas.
Also in the presence of excess potassium iodide produced iodine combines with iodide ion give red - brown I. 2 H2O aq catalase 2 H2O l O2 g hydrogen peroxide enzyme water oxygen gas. Although the cells are dead catalase still remains active.
When barium peroxide is acidified and the excess water is removed by the process of evaporation under reduced pressure we obtain hydrogen peroxide. A chemical hydrogen peroxide is shown to decompose to produce oxygen and water by capturing the gas evolved in. For example hydrogen peroxide decomposes to form water H2O and oxygen gas O2.
In this catalase and hydrogen peroxide experiment we will discover how enzymes act as catalysts by causing chemical reactions to occur more quickly within living things. Chemical properties of Hydrogen Peroxide are as follows. The second step is the catalase breaking down another hydrogen peroxide molecule by releasing oxygen gas and water.
The decomposition of hydrogen peroxide by itself is. Hydrogen peroxide as a reducing agent in the presence of a strong oxidising agent in both alkali and acidic media and importantly oxygen is released every time. We added KI to the hydrogen peroxide because KI is a known catalyst and it would speed up the reaction.
20 mL 30 hydrogen peroxide available from chemical supply establishments. As the process progresses the dishwashing soap catches the oxygen that is released by the peroxide. The resulting products are water and oxygen gas.
The final result is tons of bubbles. Decomposition of hydrogen peroxide can be catalysed by other compounds such as transition metals like silver and platinum. Why is it possible to use dead cells to study the function of this enzyme.
PbSs4H 2 O 2 aqPbSO 4 s4H 2 Ol The reducing nature in an acidic medium. As a result bottles of hydrogen peroxide you may purchase at the drug store are sold in. Adding the yeast to the hydrogen peroxide helps this breaking-down process occur much faster.
This is an experiment most students in chemistry lab are familiar with. When it comes to determining exactly why hydrogen peroxide decomposes so easily we have to look at the chemical structure of the H 2 O 2 molecule. The reaction you are referring to is the breakdown of hydrogen peroxide to water and oxygen.
The first step involves the catalase removing and binding one oxygen atom and releasing the rest of the hydrogen peroxide molecule as water. Catalase works to speed up the. Hydrogen peroxide is an oxidizing agent which reacts.
Share Tweet Send Deposit Photos Hydrogen peroxide is an unstable substance. Hydrogen peroxide is breaks down into water and oxygen. To slow this process down.
Breakdown of hydrogen peroxide. Small amounts of. Catalase breaks down and destroys hydrogen peroxide in two steps.
When ready add 1gm KI. While this is a catabolic reaction the rate at which it occurs is slow. HOClH 2 O2H 3 OClO 2.
Enzymes work by lowering the energy of activation. The decomposition of hydrogen peroxide in the presence of iodide ion occurs in two steps. Using a potato and hydrogen peroxide we can observe how enzymes like catalase work to perform decomposition or the breaking down of other substances.
H 2 O 2 aq I- aq H 2 O l OI- aq H 2 O 2 aq OI- aq H 2 O l O 2 g I- aq Materials Preparation. 50ml 30 Hydrogen Peroxide H 2 O 2 premeasured B. A pH of seven is optimal for.
Hydrogen peroxide decomposition using different catalysts. Potassium iodide and hydrogen peroxide reaction in acidic medium KI H 2 O 2 I 2 H 2 O. 9 rows Hydrogen peroxide is a colorless liquid at room temperature with a bitter taste.
Depending on the pH level. 1gm KI premeausured Procedure. Hydrogen peroxide contains a single oxygen-oxygen bond.
Hydrogen peroxide solution decomposes slowly at room temperature to water and oxygen. Potassium iodide is oxidized to iodine and hydrogen peroxide is reduced to water. The balanced chemical equation is.
However the decomposition takes place very slowly. Several measuring cylinders are set up each containing a little. Under higher temperatures and concentrations it decomposes to form water and oxygen.
That is because the enzyme. When its oxygen-oxygen bond breaks hydrogen peroxide decomposes into water and oxygen. Hydrogen Peroxide acts both as an oxidizing as well as the reducing agent in acidic and also in basic medium.
How Does A Catalyst Make Hydrogen Peroxide S Decomposition Quicker What Is Actually Happening Socratic

Question Video Using Word Equations To Describe The Decomposition Of Hydrogen Peroxide H2o2 Nagwa
Comments
Post a Comment